The ME/CFS Clinical Practice Guidelines will be developed in accordance with NHMRC’s rigorous guideline development process.
Developing the new ME/CFS Guidelines
NHMRC is leading the development of Australia’s clinical practice guidelines for ME/CFS. The guidelines will also reference related conditions including Long COVID, fibromyalgia and POTS, where they share commonalities with ME/CFS.
The ME/CFS Clinical Practice Guidelines are being funded by the Department of Health, Disability and Ageing. Further information can be found at the Hon. Minister Mark Butler’s funding announcement in June 2024.
Other NHMRC activities for ME/CFS:
- Funding for a Targeted Call for Research into ME/CFS
- ME/CFS Advisory Committee’s report to NHMRC’s CEO.
Reason for new guidelines
NHMRC has never issued or approved guidelines for ME/CFS and there are no current Australian clinical practice guidelines on ME/CFS.
What will the guidelines cover?
The new Australian ME/CFS Guidelines will aim to develop recommendations on diagnosis, management and care of people living with ME/CFS and related conditions (including Long COVID, postural orthostatic tachycardia syndrome (POTS) and fibromyalgia) based on current understanding of ME/CFS and the latest evidence most relevant to the Australian healthcare setting.
NHMRC guideline development process
NHMRC develops clinical practice guidelines through a rigorous, evidence-based process designed to ensure recommendations are trustworthy and relevant to the Australian context. This process typically involves comprehensive reviews of current scientific research, consultation with relevant experts and engagement with key stakeholders, including healthcare professionals, people with lived experience, and advocacy groups.
Draft guidelines are subject to public consultation, allowing for feedback and transparency, before final recommendations are approved and published. This approach ensures the guidelines are both scientifically sound and practical for implementation within Australia's healthcare system.
For more information about how NHMRC develops its guidelines, visit the How NHMRC develops guidelines webpage.
Timeline
An indicative timeline for the development of the ME/CFS Guidelines is provided below. The timelines for each milestone will be updated as required.
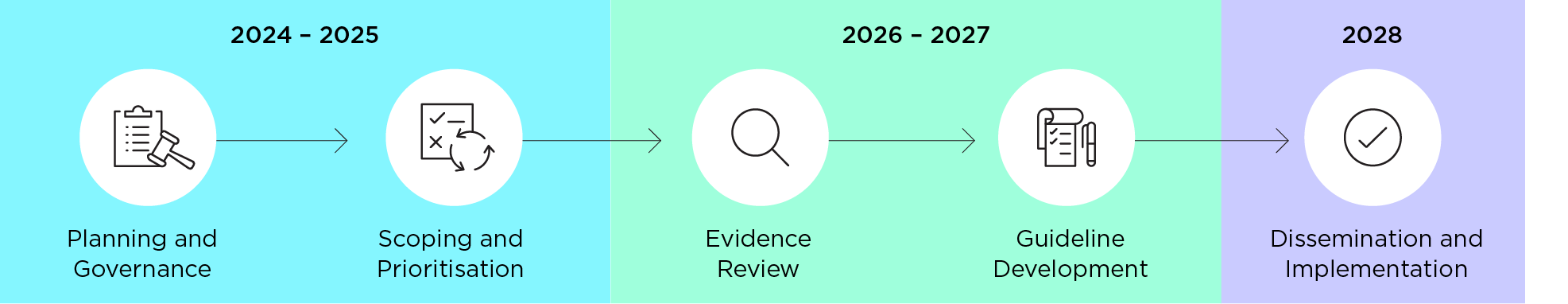
| Development stage | Expected dates | Expected timing | Status |
|---|---|---|---|
| Planning and governance | September 2024 to May 2025 | 8 months | Complete |
| Scoping and prioritisation | March to December 2025 | 9 months | In progress |
| Evidence review | October 2025 to October 2026 | 12 months | In progress |
| Development of recommendations | October 2026 to June 2027 | 8 months | |
| Draft guideline | June to December 2027 | 6 months | |
| Publication consultation / NHMRC release | August to December 2027 | 6 months | |
| Dissemination / Implementation | March 2028 | 8 months |
Visit the Governance page for more information about who will be involved in developing the ME/CFS Clinical Practice Guidelines.