Worldwide, pneumonia is a leading cause of childhood death. About one-third of these deaths could be prevented by using the pneumococcal conjugate vaccine (PCV). Most of these deaths occur in low- and middle-income countries (LMICs) that have not made full use of PCV, both because of its high cost and uncertainty about impact. Researchers at the University of Melbourne (UoM) and Murdoch Children’s Research Institute (MCRI) and their international partners have taken major steps towards making PCV more accessible globally.
Origin
Pneumonia is an acute respiratory infection that affects the lungs. The lungs are made up of small sacs called alveoli which fill with air when a person breathes. However, when a person has pneumonia the alveoli instead fill with infected fluid, making breathing difficult and limiting the amount of oxygen that can enter the bloodstream.
Pneumonia is the single largest infectious cause of death in children worldwide. In 2019, pneumonia killed about three quarters of a million children under the age of five years. This accounted for 14% of all deaths of this group and 22% of all deaths in children aged one to five years. Pneumonia affects children and families everywhere, but deaths are highest in southern Asia and sub-Saharan Africa.1
Children whose immune systems are compromised are at higher risk of developing pneumonia. A child's immune system may be weakened by malnutrition. Pre-existing illnesses, such as HIV and measles, also increase a child's risk of contracting pneumonia, as do environmental factors such as indoor air pollution and living in a crowded home.
While pneumonia may be caused by viruses, bacteria and fungi, the most common bacterial cause in children is the organism Streptococcus pneumoniae (the pneumococcus). Although efforts at vaccine development began in the early 1900s2 , the first effective pneumococcal vaccine for adults only became available in 1977, while the first vaccine designed to stimulate the infant immune system (the PCV) was released in 2000.
Because the first available versions of PCV were developed against bacterial strains most common in high-income countries, and because the vaccine has been unaffordable for LMICs, 18 LMICs are yet to introduce PCV into their national program. Countries in the Western Pacific region have been the slowest to adopt PCV into their national immunisation schedules.
PCV is one of the largest areas of expenditure for Gavi, the global alliance to increase equity in immunisation. Gavi and Gavi-eligible low-income countries spend ~US $1.5 billion on PCV over five years for three dose schedules. While the cost of PCV has declined since 2000, the prevailing understanding that three PCV doses are necessary for sustained protection meant that the absolute cost of vaccination programs remained high. Evidence was lacking that reduced-dose schedules could sustain disease control.
If using reduced-dose schedules could be shown to be effective this could be an option for eligible countries who struggle to pay for PCV, providing considerable savings while maintaining good protection.
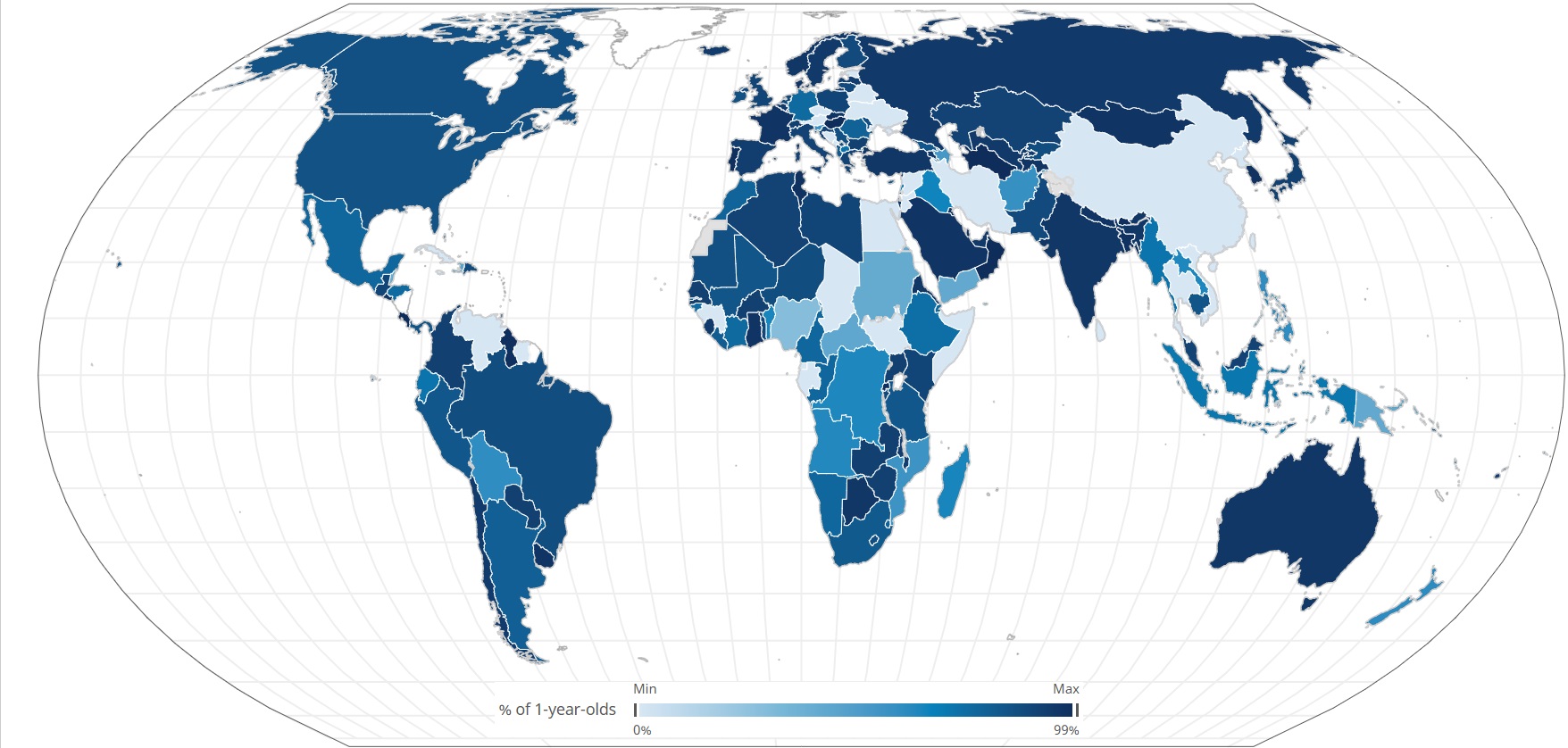
Researchers at UoM and MCRI, and their international partners including in Fiji, Vietnam, The Gambia, Mongolia and Thailand, have engaged in a long-term research program aimed at increasing PCV access globally.
Investment
NHMRC has provided funding to a number of researchers at UoM and MCRI to support research related to increasing PCV research. These include Fiona Russell, Kim Mulholland, Paul Licciardi, Catherine Satzke, Jonathan Carapetis and Claire von Mollendorf.
Key NHMRC support was provided through the Centres for Research Excellence (CRE) scheme (including for the 2012 CRE in Pneumococcal Vaccinology (CRE-PV) and the 2021 CRE for Pneumococcal Disease Control in the Asia-Pacific (CRE-PDC)) and through an NHMRC project grant for a clinical trial in Fiji.
Research has also been supported through co-funding by the US National Institute of Allergy and Infectious Diseases (NIAID), the Australian Government Department of Foreign Affairs and Trade (DFAT) and the Gates Foundation.
Other sources of funding for related research at MCRI include from The Wellcome Trust, Thrasher Foundation, Gavi, and the World Health Organization (WHO).
The PDF poster version of this case study includes a graphical timeline showing NHMRC grants provided, and other events described in the case study.
Research
In 1994, Jonathan Carapetis commenced his PhD research at the Menzies School of Health Research in Darwin, with a focus on public health, and looking at streptococcal surveillance and disease prevention in Aboriginal Australians. However, after commencing work at MCRI in 1999, Carapetis’ research began to include a focus on pneumococcal diseases. In parallel, in 2003, Kim Mulholland at UoM commenced a project to investigate alternative regimens for pneumococcal vaccination of infants in a lower-middle-income country (Fiji).
Fiona Russell joined this project as a PhD student and, supervised by Carapetis and Mulholland, had a lead role as co-Principal Investigator. The project – conducted in collaboration with the Fiji Ministry for Health and the Fiji School of Medicine and taking place from 2003 to 2009 – involved the first randomised controlled trial (RCT) comparing the efficacy of one versus two versus three doses of PCV. In Fijian infants, this RCT found one priming dose of PCV to be immunogenic, to elicit better immunological memory than two or three doses and to offer some protection against infection. The team also established that two priming doses has similar efficacy to three.
Commissioned by WHO, the MCRI team described the global age distribution of pneumococcal disease before PCV use and found that in LMICs, the youngest infants had the highest burden of disease.
Informed by the team’s pneumococcal and pneumonia disease burden data, in 2012, Fiji introduced routine infant immunisation with the 10-valent pneumococcal conjugate vaccine (PCV10). Because data were scarce for the effect of PCV in the Asia-Pacific region, the MCRI team was asked by the Fiji Ministry of Health to evaluate the effect of PCV10. This included the establishment of the invasive disease surveillance and the technology transfer of qPCR (a lab technique for studying genes and DNA) for the first time. Five years after the vaccine was introduced, hospital admissions for all-case pneumonia had fallen for children aged 24-59 months. Mortality was down by 39% among children aged 2-24 months who were admitted to hospital with all-cause pneumonia. Additionally, the team’s research on the impact of PCV on carriage and invasive disease (meningitis and sepsis) showed evidence of both direct and indirect effects on infants too young to be vaccinated and some impact on adults.
This was the first study in a middle-income country in the Asia and Pacific region to show the effect of PCV on carriage, pneumonia and invasive pneumococcal disease. This was subsequently followed by impact evaluations in Mongolia and Lao PDR using novel approaches.
The findings of the team’s trial research initiated a new stream of reduced dose schedule research undertaken at MCRI and contributed to the Gates Foundation investment in six RCTs in Asia, Africa and the UK examining two versus three PCV doses.
Of these RCTs, the Vietnam and The Gambian were led by MCRI teams. In Vietnam, the MCRI team used immunological and microbiological approaches to determine that a 1+1 schedule reduces vaccine-type carriage and provides some individual protection in the first year of life. These two pivotal RCTs were also the first to undertake head-to-head comparisons of the two PCVs available at that time, PCV10 (GSK) and PCV13 (Pfizer), including in reduced-dose schedules involving two or three doses. The Vietnam studies were highly innovative by incorporating analyses of novel correlates of protection (memory B cells), which were funded by NHMRC and Gates Foundation.
These findings have also led to the initiation of other studies led by MCRI collaborators including cluster-randomised trials in Nha Trang, Vietnam as well as in The Gambia. The Gambian cluster RCT was designed to compare a three-dose schedule (3+0) with two dose schedule (1+1) of PCV13. The MCRI team’s work on indirect effects from studies in Australia, Mongolia, Lao PDR and PNG found that PCV provides important indirect effects even in settings where vaccination rates are low and heterogeneous.
Translation
Translation of the PCV research program has particularly been enabled by the CREs. The CRE-PV extended important PCV research in high-burden settings including The Gambia, Lao PDR, Papua New Guinea (PNG), Mongolia and Indonesia.
The CRE-PDC involved a multidisciplinary research team from public health and policy, microbiology, immunology, vaccinology, paediatrics, biostatistics, genomics and health economics. Its work program comprised 10 main projects and has been an international effort with collaborators from Fiji, Vietnam, Lao PDR, Mongolia, The Philippines, Thailand, Singapore, Indonesia, Papua New Guinea and the UK.
Commissioned by WHO, the MCRI team led a number of systematic reviews and meta-analyses for the WHO Strategic Advisory Group of Experts (SAGE) on immunisation, providing the evidence base for their 2025 policy deliberations. These included:
- the impact of 3 and 4 dose PCV schedules on pneumonia, by formulation
- the impact of dose PCV schedules on pneumococcal carriage, by formulation; and
- a comparison of 2 versus 3 dose PCV schedules.
Recognising that no randomised trial evidence existed for 2-dose schedules for the most affordable PCV, the MCRI team also addressed this gap by leading the design of two RCTs in Thailand and Vietnam with respective country partners.
Anticipating the WHO policy change in 2025 to recommend 2-dose schedules for eligible countries, the MCRI team worked with the Fiji government to complete a budget impact and cost-effectiveness analysis of 2 versus 3 doses of PCV. Similar analyses are underway in Lao PDR, Thailand, and at least three other LMICs.
The success of these studies has led to further studies of the effectiveness of PCV vaccination campaigns in high-risk settings, including critical populations living in humanitarian settings in Somaliland as well as in children with severe acute malnutrition in Timor-Leste (funded through a NHMRC Clinical Trials and Cohort Studies grant).
As invasive disease and pneumonia surveillance in most LMICs either does not exist or is not of a standard that can be used for policy decisions, the MCRI team previously led the development of the WHO pneumococcal carriage guidelines which have been adopted in 76 countries. In 2025, they are leading the development of the updated WHO pneumococcal carriage surveillance guidelines.
Outcomes and impacts
MCRI’s research findings that 2 and 3 dose PCV primary series in infancy produce similar outcomes and that even a single dose may provide protection, have had global knowledge and health impact, and have led to a paradigm shift in understanding the number and timing of doses needed. These findings provided much of the evidence to support using fewer priming doses (2+1), endorsed by a WHO Position Paper change in 2012. MCRI’s research supporting fewer priming doses is now used by 84 out of 167 countries including Australia. ~90 million infants are now vaccinated with a 2+1 schedule each year. A WHO review found that 2+1 is just as effective as the standard 3+0 in reducing disease. This WHO Position Paper is used by LMICs.
MCRI’s research was instrumental in Fiji’s decision to introduce PCV (+ rotavirus and HPV vaccines) and their findings were incorporated into a DFAT child health review which formed the basis of DFAT’s investment in child health in Fiji (2011–2016).
Fiji was the first country to introduce all three vaccines at once and this prompted the first evaluation of PCV in an Asia-Pacific LMIC. UNICEF encouraged further investment in these vaccines in the Pacific. The Asia Development Bank invested ~US$25m (2018-2023) with Rotary International to support PCV’s introduction (+ rotavirus and HPV vaccines) in nine Pacific Island Countries.
MCRI’s research was a major contributor to setting the global PCV research agenda, with the Gates Foundation investing ~$30 million in clinical trials in the UK, Africa, and Asia to investigate the optimal PCV schedule using fewer doses. WHO SAGE updated its PCV Position Paper in 2025, using the findings from these trials. Countries now have access to an evidence base supporting a 2-dose schedule provided they have sustained coverage of more than 80% for five years or evidence of sustained control of pneumococcal vaccine types. For LMICs that fit this WHO criteria for a switch to two doses, this is likely to provide savings to allow improvements in coverage, or the introductions of other critical vaccines.
The United Kingdom is the only country using a 2-dose schedule, and five years following the switch from a 3-dose schedule, disease continues to be well-controlled as the UK’s coverage is >90%. The success of a two-dose schedule relies on PCV providing ongoing indirect protection for infants in the first year of life, and careful monitoring is a critical aspect of implementing this change, and that early adopter LMICs have a monitoring plan. MCRI provides a critical source of technical advice to country partners making changes to their vaccination schedules.
The regional collaborations strengthened through the CRE have provided a platform to develop the Asia-Pacific Vaccine Research Network in collaboration with the Universitas Gadjah Mada, Indonesia. This Network has been funded by DFAT. From this collaboration, MCRI and UoM are the only Australian institutions that are designated WHO Training Partner Organisations for Clinical Research Leadership Fellowships.
Researchers
Professor Fiona Russell
Fiona Russell received a PhD from UoM in 2011 and was awarded the Chancellor’s Prize for PhD Excellence. In 2018 she became a Professor at UoM. Russell is Director of the Child and Adolescent Health PhD Program, Department of Paediatrics, UoM and is a member of the WHO Collaborating Centre for Child and Neonatal Health Research and Training; and Group Leader for Asia-Pacific Health, MCRI. She is co-Chair of the World Society of Pediatric Infectious Diseases International Scientific Committee and former Chair of the Australasian Society of Infectious Diseases Vaccination Special Interest Group. Russell is founder and co-Chair of the Asia-Pacific Vaccine Research Network. During COVID-19 she was a member of DFAT’s Vaccine Access and Health Security Initiative’s Expert Advisory Group. Russell was the only Australian member of the WHO Science Division Strengthening Clinical Trials Technical Advisory Group (2023-2027) and from 2026 she will be the only Australian member of the WHO Strategic Advisory Group of Experts on Immunization.
In 2019, Russell was awarded the Australasian Society of Infectious Diseases Frank Fenner Award.
Professor Kim Mulholland
Edward (Kim) Mulholland completed medical studies at UoM in 1976. He received paediatric training at both UoM and The Royal Children's Hospital, Melbourne. He also completed post-graduate training in immunology, respiratory medicine and tropical medicine. In 1989 he joined the Medical Research Council Laboratories, Gambia. In 1995 he joined the WHO Headquarters where he oversaw the development of standardized methods for the evaluation of pneumonia vaccines in developing countries. After leaving WHO in 2000 he was one of the founders of the Global Action Plan for Pneumonia. Mulholland commenced as a Senior Principal Research Fellow at MCRI in 2003, leading the New Vaccines Research Group. From 2005 to 2011 he was also Professor of Child Health and Vaccinology at the London School of Hygiene and Tropical Medicine. Since 2020 he has been a member of the WHO SAGE on immunization.
Associate Professor Paul Licciardi
Paul Licciardi is Group Leader of Vaccine Immunology and Principal Research Fellow in the Infection, Immunity and Global Health at MCRI, and has led the immunogenicity studies of all the MCRI clinical trials on PCV schedules. His laboratory has developed significant expertise in the evaluation of PCV responses using both WHO gold standard methods as well as advanced cellular immunology technologies to define markers of long-term protection for pneumococcal disease.
Professor Catherine Satzke
Catherine Satzke leads the Translational Microbiology Group at MCRI and also led the update of WHO standards for pneumococcal carriage studies. She was an inaugural veski (Victorian Endowment for Science, Knowledge and Innovation) Inspiring Women Fellow (2016-2018) and was awarded a five-year Rebecca Cooper Fellowship (2024). Satzke received the international Robert Austrian Research Award (2012) and currently heads the award committee as a board member of the International Society of Pneumonia and Pneumococcal Diseases. Since 2000, she has been a member of the Australian Society for Microbiology (ASM) and has served as Chair of the Victoria branch. She has been appointed as a Fellow of the ASM and received the Frank Fenner award and a Distinguished Service Award. Satzke is now the President-Elect of ASM.
Professor Jonathan Carapetis AM
Jonathan Carapetis graduated from the University of Sydney with a PhD in 1998. He is Executive Director of The Kids Research Institute Australia in Perth, Western Australia, an infectious diseases consultant physician at Perth Children’s Hospital, and a Professor at The University of Western Australia.
Carapetis’ previous roles include President of the Association of Australian Medical Research Institutes (AAMRI); Director, Menzies School of Health Research, Darwin; Director, Centre for International Child Health, UoM; and Theme Director, MCRI. During the COVID-19 pandemic, Carapetis was a member of the National COVID-19 Health and Research Advisory Committee.
In 2008, Carapetis was named Northern Territory Australian of the Year. In 2018, he was appointed a Member of the Order of Australia for significant service to medicine in the field of paediatrics, particularly the diagnosis, treatment and prevention of rheumatic heart disease. In 2021 he received the Professions Award at the West Australian of the Year Awards.
Professor Grant Mackenzie
Grant Mackenzie trained clinically in Australia and completed a PhD at the Menzies School of Health Research before undertaking additional training and experience in HIV care in London and Kenya. He has been based in West Africa (The Gambia) at the Medical Research Council Unit at The London School of Hygiene and Tropical Medicine and, since 2008, at MCRI.
Dr Cattram Nguyen
Cattram Nguyen received her PhD at UoM in 2015. She has contributed to pneumococcal vaccine research over the last 10 years as the lead statistician on PCV reduced-dose trials in Vietnam and The Gambia, as well as the first post-licensure PCV impact evaluations in the Asia-Pacific. She was also the biostatistical lead in the WHO-commissioned review and meta-analysis of reduced-dose PCV schedules.
Dr Eleanor Neal
Eleanor Neal received her PhD from UoM and has worked at MCRI since 2008. Her research focuses on infectious disease modelling, vaccine evaluation, and policy translation in low- and middle-income countries, with an emphasis on pneumococcal immunisation strategy. In 2020, she was awarded the Robert Austrian Research Award for her work on mathematical modelling to predict the impact of pneumococcal vaccine schedule changes in Fiji.
Associate Professor Claire von Mollendorf
Claire von Mollendorf joined MCRI in 2017 and is currently working as a senior research fellow in MCRI’s New Vaccines and Asia-Pacific Research Groups. von Mollendorf has an honorary appointment as a Senior Fellow within UoM’s Faculty of Medicine, Dentistry and Health Sciences, Department of Paediatrics. She is the project manager for the CRE for Pneumococcal Disease Control in the Asia-Pacific (CRE-PDC).
Other researchers
Other Australian researchers whose work has supported the impacts described in this case study include those from the Asia-Pacific Health, New Vaccines, Translational Microbiology and Vaccine Immunology groups at MCRI including, Dr Eileen Dunne, Anne Balloch, Dr Ryan Toh, A/Prof Nick Fancourt, Dr Rita Reyburn, Dr Jocelyn Chan, Dr Yonatan Mesfin, Dr Joshua Szanyi, Associate Professor Natalie Carvalho, Fulgence Niyibitegeka, Dr Alicia Quach, Isatou Jagne Cox and Dr John Hart, along with their many collaborators including the Fiji Ministry of Health and Medical Services, Pasteur Institute Vietnam, Mongolia Ministry of Health, Lao Oxford Mahosot Wellcome Trust Unit, Lao Ministry of Health, University of Health Sciences Lao PDR, Papua New Guinea Institute of Medical Research, Mahidol University Thailand, London School of Hygiene and Tropical Medicine and WHO. In addition, Beth Temple and Heidi Smith-Vaughan played major roles on the VN trials.
Partner
This case study was developed with input from Professor Fiona Russell, Professor Catherine Satzke, Associate Professor Paul Licciardi, Dr Cattram Nguyen and Dr Eleanor Neal, and in partnership with Murdoch Children’s Research Institute.

References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 World Health Organization. Pneumonia [Internet]. Geneva: World Health Organization; [cited 2025 Oct 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia Accessed 8 October 2025
2 Gierke R, Wodi AP, Kobayashi M. Chapter 17: Pneumococcal Disease. Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention; 2024. Available from: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
Bibliography
Chan J, Mungun T, Batsaixan P, Ulziibayar M, Suuri B, Otgonbayar D, Luvsantseren D, Nguyen CD, Narangarel D, Dunne EM, Fox K. Direct and indirect effects of 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in children hospitalised with pneumonia from formal and informal settlements in Mongolia: an observational study. The Lancet Regional Health–Western Pacific. 2021 Oct 1;15
Dunne EM, Satzke C, Ratu FT, Neal EF, Boelsen LK, Matanitobua S, Pell CL, Nation ML, Ortika BD, Reyburn R, Jenkins K. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. The Lancet Global Health. 2018 Dec 1;6(12):e1375-85
Higgins RA, Temple B, Dai VT, Phan TV, Toan NT, Spry L, Toh ZQ, Nation ML, Ortika BD, Uyen DY, Cheung YB. Immunogenicity and impact on nasopharyngeal carriage of a single dose of PCV10 given to Vietnamese children at 18 months of age. The Lancet Regional Health–Western Pacific. 2021 Nov 1;16
Licciardi PV, Temple B, Dai VT, Toan NT, Uyen D, Nguyen CD, Phan TV, Bright K, Marimla RA, Balloch A, Huu TN. Immunogenicity of alternative ten-valent pneumococcal conjugate vaccine schedules in infants in Ho Chi Minh City, Vietnam: results from a single-blind, parallel-group, open-label, randomised, controlled trial. The Lancet Infectious Diseases. 2021 Oct 1;21(10):1415-28
Neal EFG, Chan J, Nguyen CD, Russell FM. Factors associated with pneumococcal nasopharyngeal carriage: A systematic review. PLOS Glob Public Health. 2022 Apr 11;2(4)
Neal EFG, Flasche S, Nguyen CD, Ratu FT, Dunne EM, Koyamaibole L, Reyburn R, Rafai E, Kama M, Ortika BD, Boelsen LK, Kado J, Tikoduadua L, Devi R, Tuivaga E, Satzke C, Mulholland EK, Edmunds WJ, Russell FM. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine. 2020 Jan 10;38(2):202-211
Reyburn R, Tuivaga E, Nguyen CD, Ratu FT, Nand D, Kado J, Tikoduadua L, Jenkins K, de Campo M, Kama M, Devi R. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumonia hospital admissions in Fiji: a time-series analysis. The Lancet Global Health. 2021 Jan 1;9(1):e91-8
Russell FM, Chokephaibulkit K. Will two doses of pneumococcal conjugate vaccine be enough?. The Lancet Infectious Diseases. 2024 May 1;24(5):449-51
Russell FM, Mulholland K, Greenslade L. Mass doses of fractional pneumococcal vaccine in humanitarian settings. Lancet Infect Dis. 2025 Jun;25(6):599-601. doi: 10.1016/S1473-3099(24)00768-0
Temple B, Toan NT, Dai VT, Bright K, Licciardi PV, Marimla RA, Nguyen CD, Uyen DY, Balloch A, Huu TN, Mulholland EK. Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial. The Lancet Infectious Diseases. 2019 May 1;19(5):497-509
Temple B, Tran HP, Dai VT, Smith-Vaughan H, Balloch A, Beissbarth J, Bright K, Higgins RA, Hinds J, Hoan PT, Nation ML. Efficacy against pneumococcal carriage and the immunogenicity of reduced-dose (0+ 1 and 1+ 1) PCV10 and PCV13 schedules in Ho Chi Minh City, Viet Nam: a parallel, single-blind, randomised controlled trial. The Lancet Infectious diseases. 2023 Aug 1;23(8):933-44
Weaver R, Nguyen CD, Chan J, Vilivong K, Lai JY, Lim R, Satzke C, Vongsakid M, Newton PN, Mulholland K, Gray A. The effectiveness of the 13-valent pneumococcal conjugate vaccine against hypoxic pneumonia in children in Lao People's Democratic Republic: An observational hospital-based test-negative study. The Lancet Regional Health–Western Pacific. 2020 Sep 1;2
World Health Organization. WHO position paper: Pneumococcal conjugate vaccines in infants and children aged <5 years – September 2025. Weekly epidemiological record. 2025 Sep 26; WER No 39, 2025, 100, 411–437. Available from: https://www.who.int/publications/i/item/who-wer10039-411-437. Accessed 8 October 2025