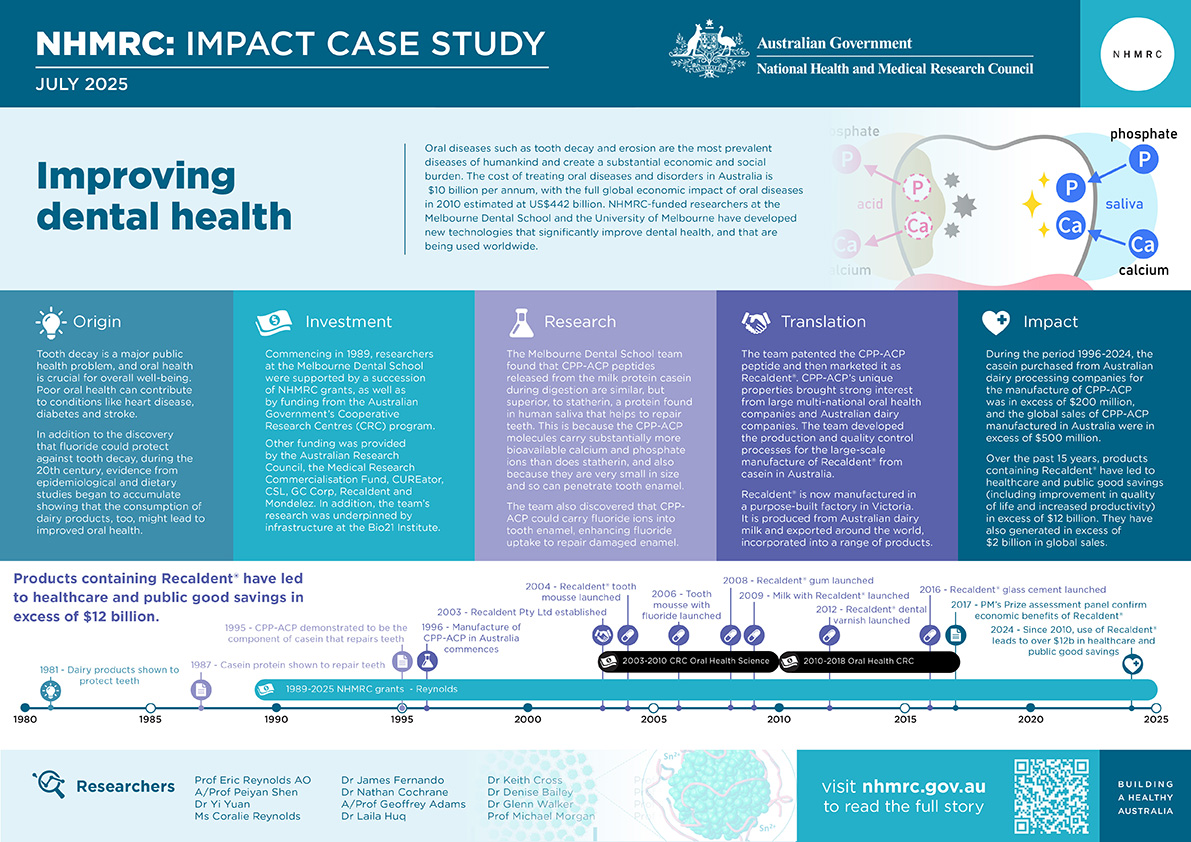
Oral diseases such as tooth decay and erosion are the most prevalent diseases of humankind and create a substantial economic and social burden. The cost of treating oral diseases and disorders in Australia is $10 billion per annum, with the full global economic impact of oral diseases in 2010 estimated at US$442 billion.1 NHMRC-funded researchers at the Melbourne Dental School and the University of Melbourne have developed new technologies that significantly improve dental health, and that are being used worldwide.
Origin
Dental caries (tooth decay or cavities) occur when tooth tissue is destroyed by acids produced by bacteria that live in a person’s mouth. By contrast, dental erosion occurs when tooth tissue is destroyed by acids or other chemicals from non-bacterial sources, such as beverages and foods, supplements or medications, or gastric acid from reflux and eating disorders. These two forms of tooth damage are connected, as eroded surfaces are porous and more susceptible to decay.
Tooth decay is a major public health problem. Currently, one in four adults in Australia have untreated caries and the rates are even higher for children.2,3 The prevalence of dental erosion is high as well, with some studies indicating 30%-70% of those examined showing signs of dental erosion.4
Oral health is crucial for overall well-being. Poor oral health can contribute to conditions like heart disease, diabetes and stroke, while a healthy mouth can improve immune function and reduce inflammation. Consequently, a key challenge for dental health-care professionals has been to find ways to treat dental caries and erosion.
The connection between fluoride and healthy teeth was discovered early in the 20th century and, since the mid-20th century, water fluoridation and fluoride-containing oral care products have provided the mainstay of oral health treatment in many countries.
Over the course of the 20th century, however, evidence from epidemiological and dietary studies began to accumulate showing the consumption of dairy products, too, might lead to improved oral health. After completing his PhD in 1978, University of Melbourne biomedical researcher Eric Reynolds began investigations into whether dairy products could provide a new and powerful method to treat and reverse tooth decay.
Investment
Commencing in 1989, the research activities of Reynolds and, over time, his increasingly large and multidisciplinary team, were continuously supported by a succession of NHMRC grants, as well as funding through the Australian Government’s Cooperative Research Centres (CRC) program.
Funding for the team’s research program also came from the Australian Research Council, the Medical Research Commercialisation Fund, CUREator, CSL, GC Corp, Recaldent and Mondelez. In addition, the team’s research was underpinned by infrastructure at the Bio21 Institute and the Melbourne Dental School.
The PDF version of this case study includes a graphical timeline showing NHMRC grants provided and other events described in the case study.
Research
Reynolds’ early research (published in 1981) demonstrated that dairy products such as milk, milk concentrates and cheeses were protective against dental damage. By 1987, Renolds and his team had also demonstrated that the exposure of tooth enamel to peptides (short chains of amino acids) found in casein – a protein making up about 80% of the proteins in cow's milk – significantly reduced tooth enamel demineralization. By 1995, the team had further demonstrated that there is a specific molecule within casein (called Casein Phosphopeptide-Amorphous Calcium Phosphate or CPP-ACP) that plays the key role in the ability of milk to repair dental caries.
The team discovered that peptides released from casein during digestion are similar to statherin, a protein found in human saliva that helps to repair teeth. However, they also found that CPP-ACP is far superior to statherin in its ability to remineralise teeth because CPP-ACP molecules carry substantially more bioavailable calcium and phosphate ions than does statherin, but are still nanosized. Consequently, they can readily diffuse through tiny pores in dental enamel to provide the building blocks for enamel crystal remineralisation and repair.
It was then shown that CPP-ACP could also carry fluoride ions into tooth enamel, thereby enhancing fluoride uptake as well, which not only helped to repair the enamel but also made it more resistant to future decay.5
These findings, supported by a number of clinical trials, suggested to Reynolds and his team that CPP-ACP-based dental care technologies could provide an enhanced ability to prevent and reverse the early stages of dental caries and erosion. Rather than dentists filling in cavities after they had formed, the use of CPP-ACP-based technologies could allow the repair of early caries and dental lesions before cavity formation had occurred.
Since they made their initial discovery, Reynolds and his team have been the leaders of a large international effort to better understand CPP-ACP. Their own international research collaborations have included researchers from Tokyo Medical and Dental University, the University of Liverpool, Queen Mary University of London, the University of Nagasaki, the University of Washington, and the University of Illinois. Domestic collaborations have included researchers from the University of Adelaide and the University of Queensland.
With further funding from NHMRC, the team also demonstrated a synergy between the CPP-ACP technology and stannous fluoride (SnF2). Stannous fluoride has become the preferred form of fluoride in toothpastes because, in addition to the decay inhibitory effects of the fluoride ion, the teeth also receive benefits from the stannous (tin) ions. These ions incorporate into the outer surface of the tooth enamel to increase hardness, helping prevent tooth erosion and abrasion. Reynolds and his team have shown that, like with calcium and fluoride ions, the CPP phosphopeptides stabilise and deliver bioavailable stannous ions to the tooth surface to enhance uptake and efficacy.6,7,8
Translation
The discovery of the CPP-ACP peptide and its unique properties brought strong interest from large multi-national oral health companies and Australian dairy companies. Because Reynolds and his team had, from the beginning, worked closely with the University of Melbourne’s commercial office to protect the intellectual property (IP) associated with their discoveries, they were able to form partnerships with these companies.
University of Melbourne trademarked the CPP-ACP nanotechnology as Recaldent® and the team then engaged in a range of further research, licensing, technology transfer, product development, clinical trials and regulatory approval processes to bring Recaldent®-based products into the market.
The team developed the production and quality control processes for the large-scale manufacture of Recaldent® from casein, and established Recaldent Pty Ltd (later acquired by Mondelez) to manufacture it. Using a purpose-built factory in Victoria, Recaldent® is produced from Australian dairy milk and exported around the world to be incorporated into a range of products.
US-based Warner-Lambert was the first oral health company to bring Recaldent® to market, introducing a sugar-free chewing gum with Recaldent® as a key ingredient. A large-scale, randomised, controlled, double-blind clinical trial (RCT) – involving 2,720 school children – demonstrated that after 24 months of chewing the sugar-free gum three times per day, there was a significant difference in caries progression/regression, compared to those children chewing a control sugar-free gum. This was the first RCT of the CPP-ACP technology.9
The trial methodology was state-of-the-art, using standardised digital radiography to measure the progression and regression of dental caries over the 24-month period. The participants were all provided with fluoride toothpastes and lived in Melbourne with fluoridated drinking water. The trial showed that the CPP-ACP technology – even on a background of optimal fluoride use – significantly slowed the progression of caries and enhanced the regression (remineralisation) of caries lesions that were present at the start of the study. This clinical trial, supported by NHMRC, established a new international benchmark for caries RCTs. This was the first conclusive demonstration of the effectiveness of the CPP-ACP technology and its results have been confirmed by other studies.
The global success of gum containing Recaldent®, with particularly strong sales in Japan, led to a collaboration between Mondelez and the Japanese company, GC Corp. Recaldent® was included in a wide range of GC’s oral products, notably their popular dental cream ‘Tooth Mousse’ and a version also containing fluoride, ‘Tooth Mousse Plus’. Clinical trials have demonstrated that these products are superior to other professionally-applied products for treating caries and erosion.
Another CPP-ACP-based product is the dental restorative glass cement FujiVII-EP. This GC product is used as a fissure sealant to protect areas at high risk of tooth decay, and to restore root caries and exposed root surfaces. Fuji VII-EP releases CPP-ACP and fluoride when exposed to acid and has been shown to protect enamel and root surfaces from the development of demineralised lesions.
A further CPP-ACP-based product is a dental varnish that is applied to teeth as a coating that releases CPP-ACP and fluoride and that is used to help prevent caries in high-risk patients. Many studies have confirmed the superiority of this CPP-ACP/F varnish over a fluoride-only varnish.
The first food product to have CPP-ACP added was ultra-heat-treated, long-life milk designated Milk de Recaldent. This product was launched in Japan in 2009. The addition of CPP-ACP to this cow’s milk product was shown by RCTs to substantially increase the content of bioavailable calcium and to increase enamel lesion remineralisation.
Outcomes and impacts
The research translation and commercial development of the CPP-ACP technology over the last twenty years has resulted in a range of products containing Recaldent® being developed and commercialised in over 50 countries worldwide. Clinical trial and global sales data for these products suggest that, over the past 15 years, they have led to healthcare and public good savings (including improvement in quality of life and increased productivity) in excess of $12 billion.9,10 They have also generated in excess of $2 billion in global sales.
The establishment of the CPP-ACP manufacturing plant in Victoria, now operated by Mondelez, and the global distribution of oral-care products containing CPP-ACP, not only resulted in a successful company generating export income for Australia, it also assisted in regional development and employment. The cost of construction of the CPP-ACP manufacturing plants at Toora and Scoresby in Victoria was over $30 million. Expenditure on the employment of plant operation, business development and R&D staff in relation to the CPP-ACP technology has been over $200 million.
During the period 1996-2024, the casein purchased from Australian dairy processing companies for the manufacture of CPP-ACP was valued in excess of $200 million, and the global sales of CPP-ACP manufactured in Australia were in excess of $500 million.
The detailed economic impact of CPP-ACP was independently confirmed by the Prime Minister’s Prize for Science assessment panel in 2017.
Researchers
Laureate Professor Eric Reynolds AO
Eric Reynolds graduated from the University of Melbourne with a Bachelor of Science (1973) then a PhD (1978). Reynolds was Head of the Melbourne Dental School and Associate Dean of the Faculty of Medicine, Dentistry and Health Sciences at the University of Melbourne (2000-2015). He is Chief Executive Officer and Research Director of the Oral Health CRC.
In 1999, Reynolds joined the Editorial Board of the Journal of Dental Research (the journal of the International Association for Dental, Oral and Craniofacial Research (IADR)). Reynolds was President of IADR in 2021/2022. He is the only Australian to be elected as President of this association which is the peak international body for dental, oral and craniofacial research, and which has over 10,000 members globally.
Reynolds received the Clunies Ross National Science and Technology Award in 2002 and the Victoria Prize for Science in 2005. Also in 2005, Reynolds was appointed an Officer of the Order of Australia. In 2011, he was awarded the IADR’s Distinguished Scientist Award. In 2016, he received the Award of Merit from the Australian Dental Association.
Other researchers
Many other researchers contributed to the development of the impacts described in this case study. They include Associate Professor Peiyan Shen, Dr Yi Yuan, Ms Coralie Reynolds, Dr James Fernando, Dr Nathan Cochrane, Associate Professor Geoffrey Adams, Dr Laila Huq, Dr Keith Cross, Dr Denise Bailey, Dr Glenn Walker, Professor Michael Morgan, Professor David Manton, Professor Stuart Dashper, Professor Laurence Walsh and Professor Michael Burrow.
Partner
This case study was developed with input from Professor Eric Reynolds and in partnership with the Melbourne Dental School and the University of Melbourne.

References
The information and images from which Impact Case Studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1. Listl S, Galloway J, Mossey PA & Marcenes W. 2015. Global economic impact of dental diseases. J. Dent. Res. 94, 1355-61
Slade GD, Spencer AJ & Roberts-Thomson KF (eds.) 2007. Australia’s dental generations: the National Survey of Adult Oral Health 2.2004–06. AIHW cat. no. DEN 165.: Canberra: Australian Institute of Health and Welfare
3. Armfield J, Slade G & Spencer A. 2006. Socioeconomic differences in children’s dental health: The Child Dental Health Survey, Australia 2001. Dental Statistics and Research Series No. 33. Canberra: Australian Institute of Health and Welfare
4. Taji S & Seow WK. 2010. A literature review of dental erosion in children. Aust. Dent. J. 55, 358-67
5. Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, Reynolds CJ. Fluoride and casein phosphopeptide-amorphous calcium phosphate. Dent Res. 2008 Apr;87(4):344-8
6. Fernando JR, Shen P, Sim CPC, Chen YY, Walker GD, Yuan Y, Reynolds C, Stanton DP, MacRae CM, Reynolds EC. Self-assembly of dental surface nanofilaments and remineralisation by SnF2 and CPP-ACP nanocomplexes. Sci Rep. 2019 Feb 4;9(1):1285
7. Al Saady Hall C, Edwards S, Reynolds EC, Richards LC, Ranjitkar S. Erosion-inhibiting potential of the stannous fluoride-enriched CPP-ACP complex in vitro. Sci Rep. 2023 May 16;13(1):7940
8. Fernando JR, Shen P, Yuan Y, Adams GG, Reynolds C, Reynolds EC. Remineralisation of enamel and dentine with stabilised stannous fluoride dentifrices in a randomised cross-over in situ trial.
J Dent. 2024 Apr;143:104895
9. Morgan MV, Adams GG, Bailey DL, Tsao CE, Fischman SL, Reynolds EC. The anticariogenic effect of sugar-free gum containing CPP-ACP nanocomplexes on approximal caries determined using digital bitewing radiography. Caries Res. 2008;42(3):171-84
10. Ran T, Chattopadhyay SK, Community Preventive Services Task Force. Economic evaluation of community water fluoridation: a community guide systematic review. American journal of preventive medicine. 2016 Jun 1;50(6):790-6.